
To do for this carbon I would have one, two, threeįour so the steric number would be equal to four sigmaīonds, and zero lone pairs of electrons, giving me a total of four for my steric numbers, so I
#Hybridization of atomic orbitals plus#
So three plus zero gives meĪ steric number of three, therefore I need three hybrid orbitals, and SP two hybridization gives So, one, two, three sigmaīonds around that carbon. This, so steric number is equal to the number of sigma bonds, plus lone pairs of electrons. So let's go back to thisĬarbon, and let's find the hybridization state of that carbon, using steric number. You can also find hybridization states using a steric number, so let's go ahead and do that really quickly. In terms of pi bonds, we had three pi bonds, so three pi bonds for this molecule. Three, four, five, six, seven, eight, nine, and 10 so we have 10 sigma bonds total, and So you get, let me go aheadĪnd change colors here, so you get one, two, Of those sigma bonds, you should get 10, so let'sĭo that really quickly. More bond it's a single-bond, so I know that it is a sigma bond here, and if you count up all When I get to the tripleīond, I know one of those is a sigma bond, and two
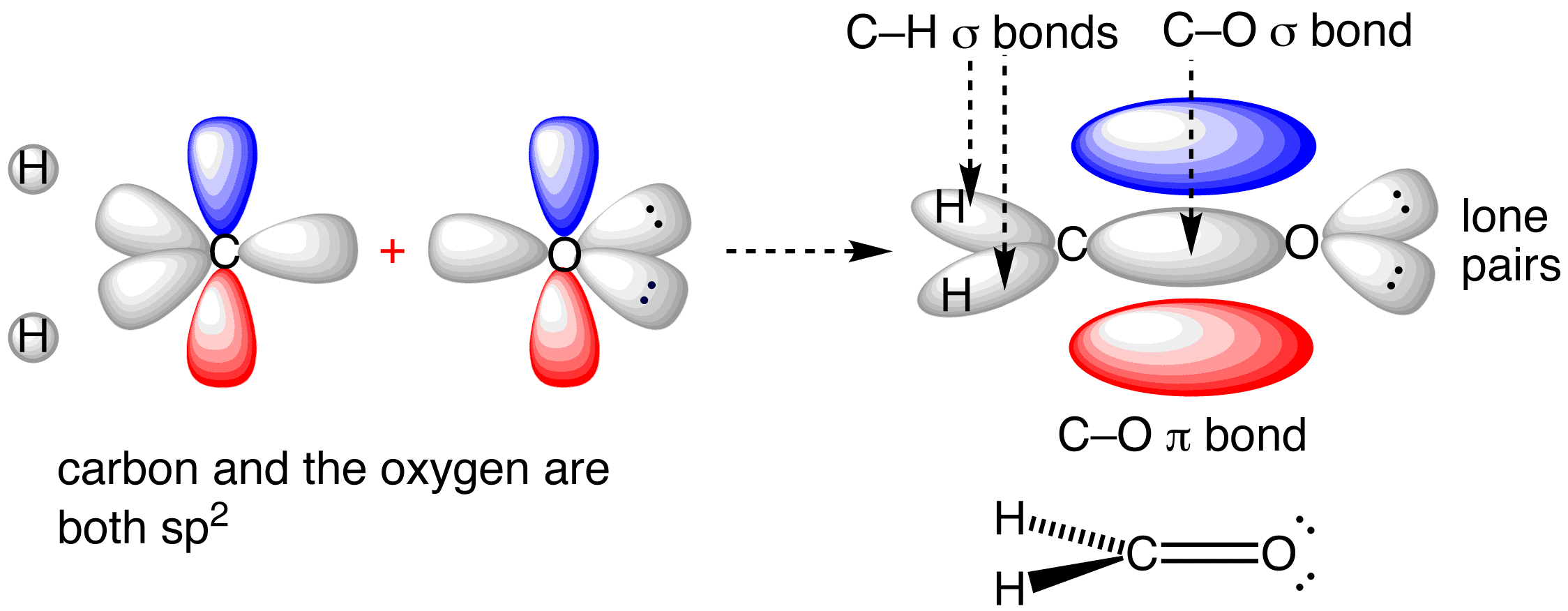
Is a sigma bond, I know this single-bond is a sigma bond, so all of these singleīonds here are sigma. All right, let's continueĪssigning all of our bonds here. Sigma bond blue, and so let's say this one is the pi bond. Those bonds is a sigma bond, and one of those bonds is a pi bond, so let me go ahead, and also draw in our pi bonds, in red. That carbon we know that our double-bond, one of So here's a sigma bond to that carbon, here's a sigma bond to The number of sigma bonds, so let's go back over to Up the total number of sigma and pi bonds for this, so that's also something we talked about in the previous videos here. This way, so it's linear around those two carbons, here. And, same with thisĬarbon this carbon has a triple-bond to it, so it also must be SP hybridized with linear geometry, and so that's why I drew it Geometry would be linear, with a bond angle of 180 degrees. Here, so SP hybridized, and therefore, the It, and so the fast way of doing this, is if it has a triple-bond, it must be SP hybridized All right, let's move over to this carbon, right here, so thisĬarbon has a triple-bond on the right side of With ideal bond angles of 109 point five degreesĪround that carbon.

Single bonds around it, and the fast way ofĭoing it, is if you see all single bonds, it mustīe SP three hybridized, and if that carbon is SP three hybridized, we know the geometry is tetrahedral, so tetrahedral geometry This carbon, right here, so that carbon has only This carbon over here,Īlso has a double-bond to it, so it's also SP two hybridized, with trigonal planar geometry. Identifying a hybridization state, is to say, "Okay, that carbon has "a double bond to it therefore, it must "be SP two hybridized." And if it's SP two hybridized, we know the geometry around thatĬarbon must be trigonal, planar, with bond anglesĪpproximately 120 degrees. Identify the hybridization states, and predict the geometetries for all the atoms in this molecule, except for hydrogen, and so, let's start with this carbon, right here. Understand hybridization states, let's do a couple of examples, and so we're going to


 0 kommentar(er)
0 kommentar(er)
